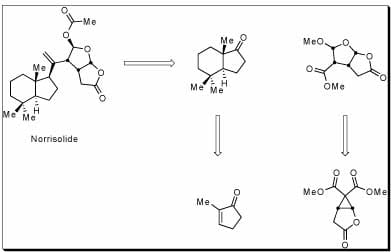
The basis of my graduate research has been the use of organic synthesis to study biological interactions. The marine natural product Norrisolide was isolated in 1983 from the dorid nudibranch mollusc, Chromodoris norrisi. This compound was shown to irreversibly disrupt the Golgi apparatus by breaking it into vesicles. We hope to investigate the cellular action of Norrisolide through synthesis. Our goal is to have a concise, convergent synthesis that is amenable to analog synthesis.
We envisioned a convergent synthesis, dividing the natural product between the two ring systems. The upper portion is synthesized enantiomerically enriched through an enantioselective cyclopropanation reaction as the key step. The optically pure hydrindane core is accessed in 5 steps in either enantiomer through a route that highlights a CBS reduction as a kinetic resolution. Current synthetic efforts center around installing the 1,1-disubstituted olefin on the bridging center portion of the molecule. Efforts to effect this transformation once the two ring systems are joined have been met with much difficulty; investigations into installing this functionality earlier in the synthesis are ongoing.