
The synthesis of natural products is fundamental to the progress of synthetic chemistry because it tests the boundaries of our technology. In our lab, we choose targets that serve as synthetic challenges in addition to having biological significance. We use our syntheses to access not only the natural product, but structural variations of the natural product which may serve as biological probes.
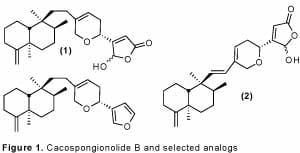
The marine sponge natural product cacospongionolide B (1) is one of a class of compounds bearing a g-hydroxybutenolide which are known to inhibit multiple forms of secratory phospholipase A2,1 enzymes believed to initiate a cascade of biological events leading to inflammation. Given the role of chronic inflammation in diseases such as asthma, psoriasis, and rheumatoid arthritis, it has become increasingly important to develop and understand the molecules which inhibit these enzymes.2
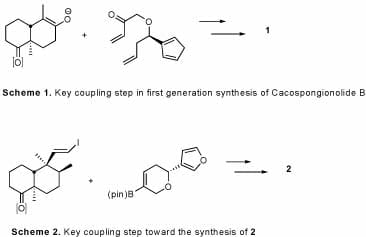
Our lab has completed 2 complementary syntheses of cacospongionolide B which have led to the development of several structural analogs. Many of these analogs have displayed increased potency over the natural product, and give insight into the natural products interaction with sPLA2.
Cacospongiolide B Publications:
- Cheung, A. C.; Snapper, M. L. “Total Syntheses of (+) and (-) Cacospongionolide B: New Insight Into Structural Requirements for Phospholipase A2 Inhibition” J. Amer. Chem. Soc. 2002, 124, 11584 – 11585.
- Cheung, A. C.; Murelli, R. P.; Snapper, M. L. “Total Syntheses of (+) and (-) Cacospongionlide B, Cacospongionolide E, and Related Analogues. Preliminary Study of Structural Features Required for Phospholipase A2 Inhibition” J. Org. Chem. 2004, 69, 5712 – 5719.
- Murelli, R. P.; Cheung, A. C.; Snapper, M. L. „Conformationally Restricted Cacospongionolide B Analogues. Influence on Secretary Phospholipase A2 Inhibition” J. Org. Chem. 2006, (in press).
- 1. Monti, M.C.; Casapullo, A.; Riccio, R.; Gomez-Paloma, L., Bioorganic & Medicinal Chemistry 2004, 12, 1467-1474.
- Heller, A.; Koch, T.; Schmeck, J.; can Ackern, K. Drugs 1998, 55, 487-496.