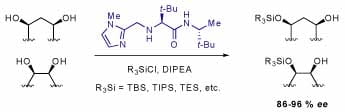
Silyl groups are one of the most (if not the most) widely used protecting groups for alcohols. Chiral catalysts can greatly facilitate the access to optically enriched organic molecules. A catalyst that promotes enantioselective silylation of alcohols, however, has not been reported. We have developed the first effective chiral silylation catalyst that initiates highly enantioselective desymmetrization of a wide range of meso-diols. The catalytic asymmetric alcohol silylations require common commercially available silyl chlorides and afford chiral silyl ethers in up to 96% ee and 96% yield. The catalyst is a simple (MW = 308 g/mole), amino acid-based organic molecule prepared in three straightforward steps from commercially available materials. Extensions of the scope of the asymmetric silylation are currently under investigation.