
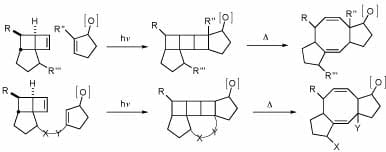
Both inter- and intramolecular [2+2] photocycloadditions of functionalized cyclobutenes have been explored in the Snapper laboratories. Thermolysis of the resulting strained cycloadducts allow for rapid entry into 5-8-5 ring systems commonly found in a variety of natural products. We are able to apply an intramolecular photocycloaddition followed by a thermolysis toward completing the total synthesis of Cycloaraneosene.
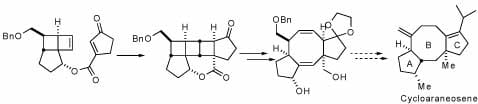
“Intramolecular [2+2]-Photocycloaddition/Thermal Fragmentation Approach toward 5-8-5 Ring Systems” Priscilla C.-K. Lo, Marc L. Snapper Org. Lett.2001, 3, 2819-2821.
“[2+2]-Photocycloaddition/Thermal Retrocycloaddition. A New Entry into Functionalized 5-8-5 Ring Systems” Michele L. Randall, Priscilla C.-K. Lo, Peter J. Bonitatebus, Jr., Marc L. Snapper J. Am. Chem. Soc.1999, 121, 4534-4535.