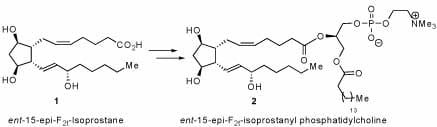
The focus of my research has been the development of stereodivergent strategies for the syntheses of a wide variety of isoprostane natural products and analogs in order to determine the biological role and cellular targets of these highly active biomolecules. Isoprostanes are an emerging class of lipid metabolites whose physiological properties are not well understood. Previously, we have synthesized eight 15-F2-isoprostanes from a streodivergent strategy. The 15-F2-isoprostanes were studied in a human whole blood platelet aggregation assay. It was discovered that the unknown ent-15-epi-F2t-isoprostane (1) was more active than the known 15-F2t-isoprostane by an order of magnitude. This new activity inspired us to design a more selective synthesis. A stereo- and enantioselective synthesis of ent-15-epi-F2t-isoprostane (1) was developed. The key sequence involves a regioselective olefin cross metathesis of a highly functionalized, enantiomerically enriched divinyl cyclopentyl intermediate. This route has allowed us to synthesize other derivatives, including an isoprostanyl phospholipid (2) – a possible intermediate in the biosynthesis of the isoprostanes.