
Research in the Snapper group has introduced several new transformations based on intramolecular cycloadditions of cyclobutadiene. These type of cycloadditions produce very rigid and compact ring systems. Further functionalization of these strained compounds provides the opportunity to generate libraries of compounds with novel molecular geometries. These diverse libraries represent unexplored chemical space and biological screening may lead to potent new interactions due to the unique presentation of interactive functionality.
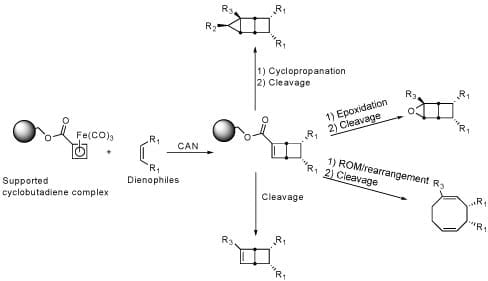
Generation of these libraries begins with the tricarbonylcyclobutadiene iron complex tethered to a solid support (1). This facilitates parallel synthesis and purification. Oxidation of the supported complex in the presence of various dienophiles provides the parent bicyclo[2.2.0]hexene library (2) which is then further functionalized into several daughter libraries.