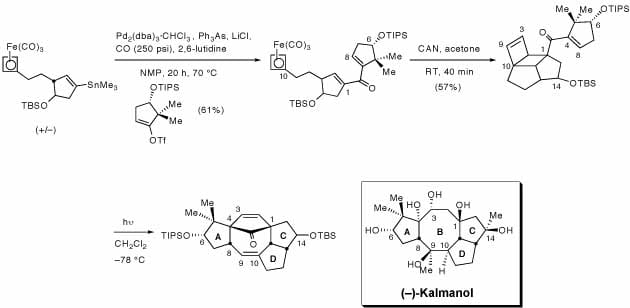
Kalmanol, a diterpenoid, possesses cardiotoxic activity and the unusual fused 5-8-5 ring system with ten stereocenters, six of which are populated with hydroxyl groups, has prompted synthetic studies on kalmanol and related molecules. We have recently completed the construction of the fused 5-8-5 ring system by an intramolecular [2+2]-photocycloaddition of a cyclobutenone-tethered cyclobutene to introduce the necessary functionality at the desired ring junctures. The resulting strained photoadduct then fragments to provide the dialkenyl cyclobutane suitable to undergo a Cope rearrangement and yield the 5-8-5 ring system. This novel strategy allows for access to the tricyclic core found in a number of natural products in as few as six steps and in an effective manner. Although a number of attempts have been made to synthesize kalmanol, none have been succcessful. Effort towards the functionalization of the double bonds will be crucial to introduce the remaining hydroxyl functionalities.