
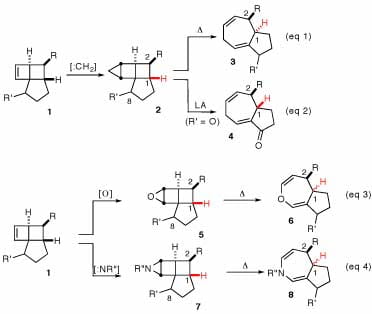
Our research has centered on the development of new methodologies for the synthesis of bicylco[5.3.0]decanes: a cyclopropanation-ring expansion strategy of the highly strained cyclobutenes (1) was discovered in our laboratories. We have reported two separate fragmentation strategies that provide complementary stereochemical outcomes (eq 1 & 2).1 Currently we are showcasing this methodology in the total synthesis of several terpenoid natural products. We are also investigating new epoxidation-ring expansion (eq 3)2 and aziridination-ring expansion (eq 4) strategies to generate oxepine and azepine 5-7 ring systems.
- Thermal: (a) Deak, H. L.; Stokes, S. S.; Snapper, M. L. J. Am. Chem. Soc. 2001, 123, 5152. Lewis Acid: (b) Deak, H. L.; Williams, M. J.; Snapper, M. L. Org. Lett. 2005, 7, 5785.
- Leyhane, A. J.; Snapper, M. L. Org. Lett. 2006, 8, 5183.