The major effort of our research program has centered on the development of new catalytic systems for stereoselective chemical transformations and their applications for practical synthesis of biologically and pharmaceutically important molecules. We are particularly interested in developing one-electron catalytic approaches to harness the vast potential of homolytic radical reactions for stereoselective construction of molecular structures. To this end, we have been in the process of establishing metalloradical catalysis (MRC) as a new concept and a general approach for controlling reactivity and selectivity of various radical processes. For achieving enantioselective radical reactions, we have developed a family of unique chiral metalloradical catalysts based on structurally well-defined Co(II) complexes of D2-symmetric chiral porphyrins with tunable electronic, steric, and chiral environments. These Co(II)-based metalloradical catalysts have been shown to be highly effective for a wide range of stereoselective radical reactions, including C=C cyclopropanation, C=C aziridination, C–H alkylation and C–H amination. Due to their distinctive radical mechanisms that involve unprecedented α-metalloalkyl radical (LnM–C•R2) and α-metalloaminyl radical (LnM–N•R) intermediates, the Co(II)-based metalloradical systems enable addressing some long-standing challenges associated with these important organic transformations. We are currently in a position to explore further applications of MRC for stereoselective organic synthesis such as catalytic C=C epoxidation and C–H hydroxylation reactions via α-metalloxyl radical intermediates (LnM–O•) as well as to develop radical cascade processes for stereoselective synthesis of complex organic molecules. In addition, we have been pursuing a related effort toward heterogenizing these homogeneous catalytic systems via construction of metal-porphyrin frameworks (MFPs) and other approaches.
Radical Reactions for Organic Synthesis: Opportunities and Challenges
In principle, radical chemistry is as rich as ionic chemistry. In addition to substitution and addition reactions, radical chemistry features several types of unique reactions such as radical atom abstraction and radical β-scission. Radical chemistry has attracted growing research interest over the last few decades and radical reactions have now been well recognized as potentially powerful tools for organic synthesis. In fact, their synthetic applications have already been demonstrated in many fields, including in the synthesis of complex natural products. In comparison with ionic reactions, radical reactions have a number of inherent synthetic advantages. For example, they typically proceed at fast reaction rates under mild and neutral conditions in a broad spectrum of solvents and show significantly high functional group tolerance. Furthermore, radical processes have the capability to perform in a cascade fashion, allowing for the rapid construction of complex molecular structures with multiple stereogenic centers.

Organic synthesis has been dominated by chemical reactions that are based on two-electron processes, either stoichiometrically or catalytically. Despite the aforesaid vast potential and great opportunities, application of one-electron radical reactions in organic synthesis has been hampered by several long-standing challenges. In an effort to further enhance the synthetic applications of radical reactions, we have been interested in developing fundamentally new approaches for effective control of their reactivity as well as stereoselectivity, especially enantioselectivity, a challenging issue that is intrinsically associated with the “free” nature of radical chemistry.
The Concept of Metalloradical Catalysis: New Approach for Catalytic Radical Processes
Most known catalytic processes are based on the employment of catalysts with close-shell electronic structures, either as transition metal complexes or small organic molecules, that proceed with catalytic mechanisms consisting of two-electron elementary reactions. We envisioned the possibility of addressing the aforementioned challenges of radical reactions through catalysis by developing open-shell transition metal-based catalysts that proceed with one-electron catalytic mechanisms. Among open-shell paramagnetic metal complexes, the simplest cases are those having electronic configurations with only single unpaired electron in a well-defined d orbital, which are called metalloradicals. Considering the inherent reactivity associated with the unpaired electron, we hope to identify one-electron activation pathways for common organic molecules by metalloradical-based catalysts to generate corresponding intermediates. With appropriate combination of the transition metal ion with desired oxidation state and the supporting ligand having suitable coordination environment, it is hypothesized that the original M-based radical character could be completely transferred to the organic precursor upon activation, resulting in catalytic generation of C–, N– or O–based radicals. These radical intermediates would function in a similar way as free organic radicals with capability of the common radical reactivities. But they are not “free” since they remain bonded covalently by the metal complex. Consequently, their subsequent radical reactions may allow for effective control of reactivity and selectivity by the nature and environment of the bonded metal complex. By taking advantage of relatively weak M–C, M–N, and M–O bonds, we hope to turn over the one-electron catalytic process for selective formation of desired products.
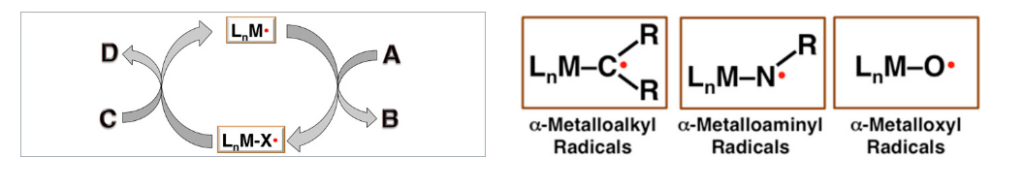
With its successful demonstration for several important chemical transformations, we are currently in the process of defining and formulating the intension and extension of metalloradical catalysis (MRC) as a general concept. Essentially, MRC entails the use of open-shell transition metal complexes as metalloradical catalysts to facilitate and control chemical reactions through catalytic transfer of radical character. It concerns catalytic processes involving radical mechanisms that are consisted of one-electron elementary steps. MRC represents a fundamentally new approach to control reactivity and selectivity of radical reactions. It provides a potential solution to perform enantioselective radical reactions.
