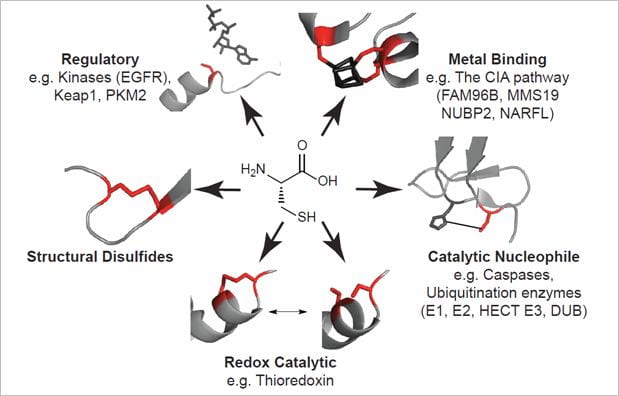
Our research program at Boston College applies chemical probes and mass spectrometry-based proteomics to investigate and perturb protein activities in complex proteomes. In particular, we focus on understanding the role of cysteine-mediated protein activities in cancer and degenerative diseases associated with aging. Cysteine-mediated protein activities comprise proteases, oxidoreductases and metabolic enzymes that rely on cysteine residues for catalysis and regulation. These functional cysteine residues demonstrate heightened reactivity relative to non-functional cysteines, and are sensitive to a myriad of oxidative protein modifications that serve to regulate protein activity in vivo. In cancer and age-related degenerative diseases, the cellular redox homeostasis is severely disrupted and the resulting oxidative stress can have dramatic consequences on cysteine-mediated protein activities.
We apply two chemical proteomic approaches to investigate cysteine-mediated protein activities: 1). An activity-based proteomic approach to report on reactivity changes in hundreds of cysteine residues concurrently, and 2). A chemical genetic platform to identify chemical agents that selectively perturb subsets of these cysteine-mediated protein activities. Through these multidisciplinary approaches that encompass aspects of synthetic chemistry, cell biology, proteomics and mass spectrometry, we can identify therapeutic targets and small-molecule drug-leads for the diagnosis and treatment of diseases such as cancer. Our functional proteomic methods are of general value for the analysis of many patho(physiological) processes, and current and future collaborations will enable the application of these tools to a diverse array of biological problems.