- Introduction
- Energy-Conversion Heterogeneous Catalysis
- Solar-to-Chemical Energy Conversions
- Fabrication of Nanomachines
- Instrumentation

Introduction
The ultimate goal of our research program is to develop high-performance catalysts to respond to the current energy crisis. Our principal strategy is to precisely control the architecture and composition of the materials at the nanoscale by the means of wet chemistry and vapor-phase deposition techniques. The projects are categorized into two directions: energy conversion heterogeneous catalysis and sunlight-driven photocatalysis. The heterogeneous catalysis studies aim to improve the stability, activity, and selectivity of heterogeneous catalysts for important industrial-type catalytic reactions. The target reactions include syngas reactions, biomass reactions, and low-temperature PEM fuel cell reactions. We also perform thorough in-situ catalytic studies over our materials via lab-based and synchrotron-based techniques. The in-situ studies of the evolution of catalysts under the reaction conditions are crucial to understand the catalytic behaviors. The photocatalysis studies aim to establish new synthetic strategies of nano-photocatalysts for optimizing solar-to-chemical energy conversions.
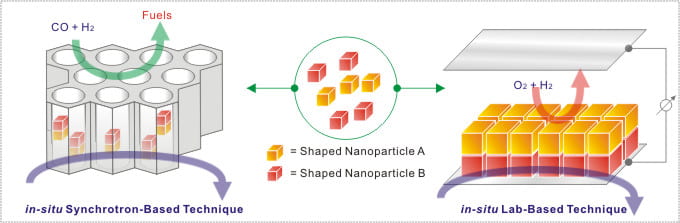
Energy-Conversion Heterogeneous Catalysis
Heterogeneous catalysis is critical for the prosperity of human civilization. It provides access to the range of chemicals, materials, and fuels we use. Understanding and controlling catalytic processes are also essential for developing improved energy storage and conversion technologies. Unfortunately, the rational design of heterogeneous catalysts is challenging because it is difficult to identify, characterize, and tune the active sites. We study new approaches to enable the molecular-level design of active sites in heterogeneous catalysts. These precise active sites allow fundamentally tuning of molecule sorption behaviors, reaction pathways, and the corresponding rates.
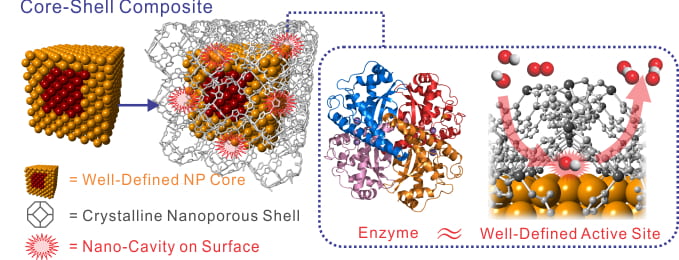
Metal-Organic Framework and Zeolite Coating
Towards our long-term vision of precisely controlling active sites, we have pioneered methods to coat metal nanoparticle catalysts with crystalline nanoporous materials, metal-organic-framework (MOF) and inorganic zeolite-based shells. Metal nanoparticles are widely used as catalysts in for petrochemical processes, industrial fine chemical synthesis, and fuel cells. Our hypothesis is that the precise molecularly-defined pores intrinsic to the MOFs and zeolites will provide a new mechanism to control the interaction between reactants undergoing catalytic transformations on the surface of the metal particle. We combine composition and facet controlled metal nanoparticles with precisely tuned pore structures to manipulate the diffusion, sorption, orientation, and conformation of the reactant molecules during the reaction.
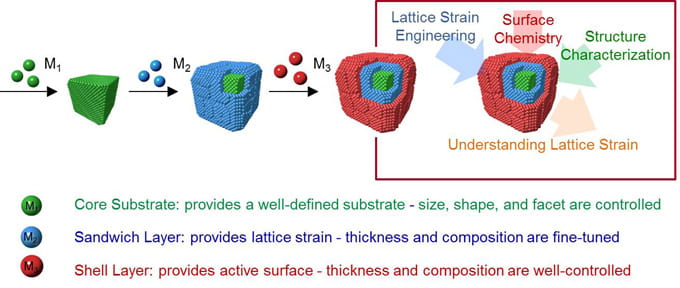
Lattice Strain Controlled Heterogesous Catalysis
A second unexplored mechanism to control the electronic structure of active sites on nanoparticle surfaces is to fine-tune the lattice strain. Our hypothesis is that the strain will alter the adsorbed reactant binding energy and thus shift the reaction pathway. We have developed several new colloidal synthetic strategies to engineer well-controlled metal-metal interfaces in a single nanoparticle. We synthesized platinum, palladium, gold, and rhodium nanoparticles with twinned, core-shell, and nanoscale-phase-separated cage-structures. By carefully tuning interaction between the different metals in a nanoparticle we tuned the degree and direction of lattice strain. We showed the lattice strain modifies chemical reactivity, for instance increasing the rate fuel-cell-related electrochemistry reactions.
Long-Term Goal
The long-term goal of our research program uncovers both the design principles that govern catalytic reactions and the synthetic tools that will enable production of high-performance designer nano-catalysts. Ultimately, these high-performance catalysts will be designed with all non-precious earth-abundant metals. Most catalysts used in industry contain platinum or other precious metals such as rhodium and palladium. The ability to independently tune reactant binding through the incorporation of precise porous coatings, combined with the ability to tune the electronic structure of the catalyst surface by strain-engineering, provides a toolkit to control activation energies of rate determining steps and overall reaction rates on precision metal surfaces. Achieving this goal will have a significant impact on the field of heterogeneous catalysis, as well as on the society as a whole.
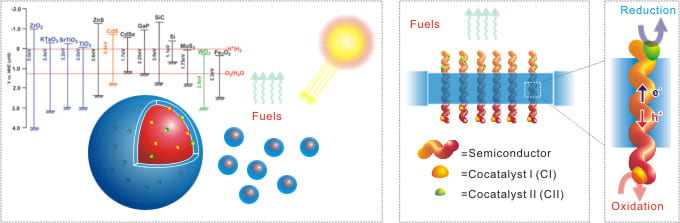
Solar-to-Chemical Energy Conversions
Solid-state photoelectrocatalytic (PEC) systems generally consist of a semiconductor (SC) and co-catalysts. The SC absorbs sunlight and separates photon-generated electron/hole pairs, while the co-catalytic sites promote the desired charge-transfer redox reactions. There are several fundamental requirements for PECs to provide adequate performance. First, the bandgap of the absorber must be greater than or equal to the thermodynamic energy required to drive the reduction and oxidation half reactions, plus the overpotential required for each. In addition to the bandgap requirement, energetics of the semiconductor surface band edges must be such that the electrons in the conduction band possess an electrochemical potential energy greater than that needed to reduce the reductant and the holes at the valence band posses an energy less than that needed to oxidize the oxidant. Third, the SC must be stable under operating conditions. Unfortunately, only few of the known cost-effective materials meet all the requirements. Overcoming this limitation is a major motivation for the projects of this direction..
To design a nanocomposite photocatalytic system
Traditionally there are two basic configurations for PEC systems: photoelectrode systems and particle systems. We focus on particle-PEC systems and further engineer these systems on the nanometer scale. The overwhelming advantages of the nano-PECs arise from their small dimensions. The nanoparticle size can be chosen such that the diameter is much smaller than the diffusion length to minimize the energy losses due to the electron/hole recombination. The decreased photon absorption in a single particle can be overcome by simply increasing the number of particles available to absorb light. In the nano-PEC system, the overpotential required for the half reactions can be surmounted by increasing the surface kinetic energy through addition of oxidation and reduction nanoparticle co-catalysts to the SC surface, thereby maximizing the efficiency.
To develop high-surface-area photocatalyst film system for visible-light driven water splitting
We also concentrate on developing new synthetic strategies by integrating sol-gel chemistry and vapor-phase deposition for high-surface-area semiconductor films with diverse compositions. Ultimately, the target is to utilize these synthetic strategies for developing photocatalyst films for visible-light-driven water splitting.
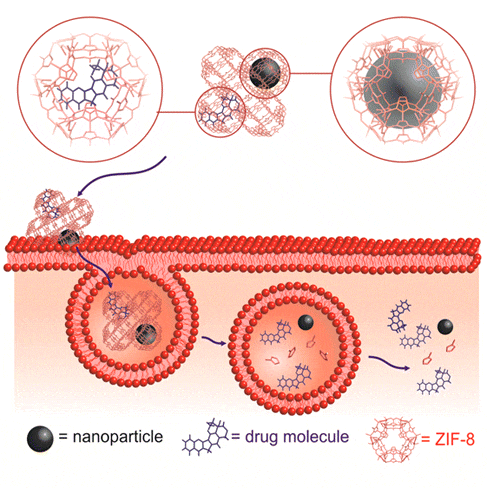
Fabrication of Nanomachines
Drug and molecule delivery has been an attractive topic in nanoscience for decades. In most of the conventional designs, the nanoparticles only served as a carrier and the motion of the carriers were driven by external energy sources, such as a strong external magnetic field. In our design, we synthesize nanosized heterostructural carriers with well-controlled structures, which allow us to control catalytic behaviors of our nanomachines. Thus, the motion of our nanomachine carriers is driven by decomposing fuels around the carriers without any external energy source
Instrumentation
Transmission electron microscopes (TEM), scanning electron microscopes (SEM), gas chromatography (GC), mass spectrometry, potentiostats, solar simulator, X-ray photoelectron spectroscopy (XPS), atomic force microscope (AFM), X-ray diffraction spectrometer (XRD), porosimiters, nuclear magnetic resonance (NMR), UV-Vis and IR spectrometer, confocal microscopy, thermogravimetric analysis and differential scanning calorimetry (TGA/DSC), zeta potential measurements, dynamic light scattering, in-situ synchrotron-based XPS, extended X-ray absorption fine structure (EXAFS), and Transmission X-ray Microscopy (TXM).
